cr3 electron configuration|Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) : Manila Key Takeaways: Chromium’s electron configuration is [Ar]3d^5 4s^1. It contains a total of 24 electrons divided among different orbitals and subshells. The electron configuration . Randomuser1285's profile page. EroMe is the best place to share your erotic pics and porn videos. Every day, thousands of people use EroMe to enjoy free photos and videos. Come share your amateur.According to the outright Scottish Premiership betting, Celtic are odds-on favourites to win the title in 2024/25.The Bhoys are defending champions after finishing on 99 points last season, winning 32 of their 38 matches. Ange Postecoglou has left for Tottenham, but his replacement, Brendan Rodgers, is a familiar face in Glasgow.
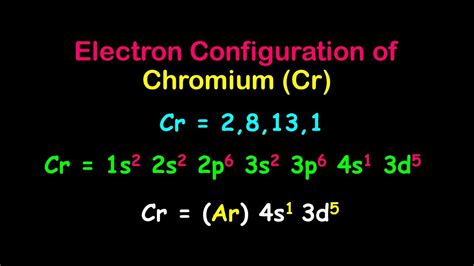
cr3 electron configuration,How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are simple.Learn how to write the electronic configuration of chromium and its ion Cr 3+ using the d-block notation. Find out why Cr 3+ has a different configuration from other d-block elements.cr3 electron configuration Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons .
Key Takeaways: Chromium’s electron configuration is [Ar]3d^5 4s^1. It contains a total of 24 electrons divided among different orbitals and subshells. The electron configuration .Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of .
First, write the electron configuration for the neutral atoms: Zn: [Ar]3d 10 4s 2; Cr: [Ar]3d 5 4s 1; Next, remove electrons from the highest energy orbital. For the transition metals, electrons .
The Cr atom consists of 24 electrons, out of which 18 come under Argon gas configuration, and the remaining three are allocated in 4s orbital with 1 electron and 3d .cr3 electron configurationHow to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are simple.
Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) Explanation: The atomic number of Chromium is Z = 24, therefore a Cr atom possesses 24 electrons. Cr:1s22s22p63s23p64s13d5. Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons.
The arrangement of electrons in chromium in specific rules in different orbits and orbitals is called the electron configuration of chromium. The electron configuration of chromium is [ Ar] 3d 5 4s 1 , if the electron arrangement is through orbitals. Electron configuration can be .
What is the electron configuration of Cr 3 +? Solution. Chromium: Chromium is a d-block element having atomic number 24. The chemical symbol of Chromium is ‘Cr’. Electronic configuration of d-block. In general, the electronic configuration of these elements is ( n - 1) d 1 - .
To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons for the Cr atom.Key Takeaways: Chromium’s electron configuration is [Ar]3d^5 4s^1. It contains a total of 24 electrons divided among different orbitals and subshells. The electron configuration determines the stability of Chromium’s valence electrons. Chromium ions have different electron configurations due to the loss of valence electrons.Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.

By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table.

First, write the electron configuration for the neutral atoms: Zn: [Ar]3d 10 4s 2; Cr: [Ar]3d 5 4s 1; Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. For the p-block elements, electrons are removed from the p orbitals and then from the .
The Cr atom consists of 24 electrons, out of which 18 come under Argon gas configuration, and the remaining three are allocated in 4s orbital with 1 electron and 3d orbital with 5 electrons. chromium unabbreviated electron configuration. The unabbreviated electronic configuration of the chromium atom is 1S22S22P63S23P63d54S1.How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are simple. Explanation: The atomic number of Chromium is Z = 24, therefore a Cr atom possesses 24 electrons. Cr:1s22s22p63s23p64s13d5. Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. The arrangement of electrons in chromium in specific rules in different orbits and orbitals is called the electron configuration of chromium. The electron configuration of chromium is [ Ar] 3d 5 4s 1 , if the electron arrangement is through orbitals. Electron configuration can be .What is the electron configuration of Cr 3 +? Solution. Chromium: Chromium is a d-block element having atomic number 24. The chemical symbol of Chromium is ‘Cr’. Electronic configuration of d-block. In general, the electronic configuration of these elements is ( n - 1) d 1 - . To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons for the Cr atom.Key Takeaways: Chromium’s electron configuration is [Ar]3d^5 4s^1. It contains a total of 24 electrons divided among different orbitals and subshells. The electron configuration determines the stability of Chromium’s valence electrons. Chromium ions have different electron configurations due to the loss of valence electrons.Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table.
cr3 electron configuration|Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH0 · What is the electron configuration of Cr 3+? Chemistry Q&A
PH1 · What is the electron configuration of Cr 3+?
PH2 · Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH3 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH4 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH5 · Electron Configuration For Chromium
PH6 · Chromium Electron Configuration: 9 (Easy Step
PH7 · Chromium
PH8 · 9.6: Electron Configurations of Ions
PH9 · 3.1: Electron Configurations